Density
- Density is the mass, or the amount of material, that a given volume of a substance contains. The density of fumed silica is 2.2 grams per cubic centimeter while of marble calcium carbonate the bulk density is 2.72 g/cm3. Fine-grained clayey–calcareous silicite may have other values.
What is Bulk density?
Bulk density is defined as the weight per unit volume of material.Texture and organic matter are major modifiers of bulk density.This properties affect water infilteration rate, movement and water retention in soils which inturn affect water soil erosion.
When is the particle density equal to the bulk density?
This would be true of a perfectly uniform, homogenous material.
Normally soil are not uniform as result of many soil management practices on properties or different vegetation and slope besides mineral content,pores,organic matter distribution. For example calcareous soils and silica sand (2.2)has different density.Based on index properties measurements made on board the JOIDES Resolution, we(Franck C. Bassinot,2 Janice C. Marsters,3 Larry A. Mayer,4 and Roy H. Wilkens3) studied porosity changes with depth in the fairly homogeneous deep-sea calcareous sediments cored during Ocean Drilling Program Leg 130 on the Ontong Java Plateau.Thus porosity will be reflected in bulk density as well.
Study USLE and Influence oF Calcarious Soils
Soil erodibility defines the resistance of soil to detachment by rainfall impact and/or surface flow force. In the Universal Soil Loss Equation (USLE), the soil erodibility (K) is estimated using the texture, organic matter content, permeability and structure of a soil.
The USLE was originally developed for non-calcareous soils in the USA. However, in calcareous soils, calcium is an important factor affecting soil structure and hence may influence soil erodibility. The application of the USLE to calcareous soils therefore requires a reassessment of K. The present study evaluates K and identifies factors affecting K for calcareous soils in Hashtrood City, northwestern Iran. The soils contain 13% lime and 1% organic matter, and are mainly utilized for wheat dry farming. A square agriculture area of 900 km2 was selected and then divided into 36 grids of 5 × 5 km. The erosion unit plots at three replicates with 1.2 m spacing were installed in each grid. K was measured based on soil loss and the rainfall erosivity index from March 2005 to March 2006. The rate of soil loss resulting from 23 natural rainfall events during the study period was measured at the unit plot scale. Various soil properties including the contents of sand, silt, silt + very fine sand, clay, gravel, organic matter, lime, and potassium as well as aggregate stability and permeability were measured in the vicinity of each plot. The results show that K significantly correlates with the contents of sand, silt, silt + very fine sand, organic matter, and lime as well as water-aggregate stability and permeability. The application of principal component analysis (PCA) also indicates that the contents of clay and lime as well as permeability strongly control K. The contents of clay and lime, which have not been well considered in USLE studies, significantly decrease K due to their strong effects on aggregate stability and water infiltration into soil. K can be estimated using a linear regression equation based on the contents of sand, clay and lime.
The influence of many soil manegement practices
The influence of many soil manegement practices on properties that affect water movement and water retention in soils. Because texture texture and organic matter are major modifiers of bulk density,their effect needs to further quantified as to evaluate the influence on erosion
A=RKLSCP
A = average annual soil loss in the project or farm area.
R = rainfall erosivity index
K = soil erodibility factor
L = topographic factor, L for slope length
S = topographic factor, S for percent slope
C = a cropping-management factor
P = conservation practice factor
 |
| Making Terrace is to reduce slopen length L |
 |
| At Mid Slope S is small but without cover C factor is high.This generate splash erosion and run off |
 |
| Increasing Run off volume gain momentum (kinetic enenergy) as run of flow down slope |
 |
| Steeper slope generate greater kinetetic energy of runoff |
View From Tikam Batu
 |
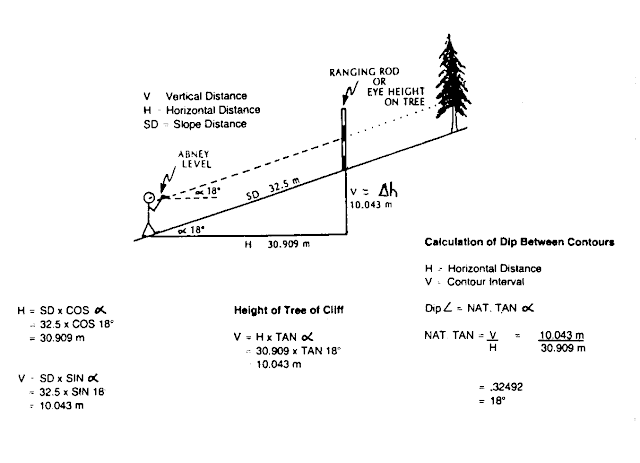
| Abney Level |
soil organic matter
Examination of data on North Wales soils shows that a good correlation exists between loss-on-ignition and organic C values, determined by Tinsley's method. Ignition for half an hour at 850° C, and for 16 hours at 375 ±5° C have both been employed. The latter has advantages over the former procedure. Regression lines and prediction limits for organic C from loss-on-ignition are given from the data obtained. Although these regressions are not necessarily expected to be generally applicable, examination of some published data suggests that closely similar expressions may be. The method, because of its simplicity, can be usefully applied in a wide range of survey, analytical and ecological studies, in spite of the known sources of error.Two gravimetric procedures for determining carbon in soil, one a dry combustion method and the other a wet oxidation method, were compared with seven variants of the titrimetric method, all based on titrimetric determination of the dichromate consumed when soil is heated with dichromate and acid. The coefficients of variation of the methods were, as percentages: dry combustion, 0.76; wet combustion, 1.1; Tinsley I, 1.3; Tinsley II, 1.8; Tinsley III, 0.8; Anne, 1.3; Mebius, 1.8; Walkley and Black, 1.6 and Tyurin, 8.5. Taking the dry combustion method as standard, the percentage recovery of organic carbon from 22 soils was 99 for wet combustion, 95 for Tinsley I, 95 for Tinsley II, 97 for Tinsley III, 93 for Anne, 95 for Mebius, 77 for Walkley and Black and 93 for the Tyurin method. A variant of the Tinsley method (Tinsley III) is proposed as a quick procedure when the accuracy of dry combustion is not essential.
PRINCIPLE
1. In this reaction carbon is oxidized by the dichromate ion. Excess dichromate ion is then back titrated with ferrous ion.
a. Dichromate ion reacts with carbon as follows:
Cr2O72 3Co+ 16H+ 4Cr3+ + 3CO2 + 8H2O
b. Ferrous ion reacts with dichromate as follows:
6Fe2+ + Cr2O72 + 14H+ 2Cr3+ + 6Fe3+ + 7H2O
REAGENTS
1. Potassium Dichromate: K2Cr2O7
2. Ferrous Ammonium Sulfate: Fe(NH4)2(SO4)26H2O
3. Sulfuric Acid: H2SO4
4. Phosphoric Acid: H3PO4
5. Sodium Fluoride: NaF
6. Diphenylamine: C6H5NHC6H5
SOLUTIONS
1. 1N Potassium Dichromate: a. Weigh 49.04 g potassium dichromate (previously dried for 2 hours at 100 C) into a 1 liter volumetric flask. Dissolve and dilute to volume with deionized water and mix well.
2. 0.5N Ferrous Ammonium Sulfate
a. Slowly add 20 ml. sulfuric acid to a 1 liter volumetric flask containing 800 ml. deionized water.
b. Add 196.1 g ferrous ammonium sulfate. Dissolve, dilute to volume with deionized water, and mix well.
c. Prepare daily.
3. Diphenylamine Indicator:
a. Dissolve 0.500 g diphenylamine in 20 ml. deionized water.
b. Slowly add 100 ml. sulfuric acid. Carefully mix with a glass stirring rod. CAUTION: this solution is corrosive and can cause sever burns. Proper precautions are given on the MSDS sheet for sulfuric acid.
PROCEDURE
1. Weigh 1.00 g soil into a 500 ml. erlenmeyer flask.
2. Add 10 ml. of 1N potassium dichromate solution.
3. Add 20 ml. sulfuric acid and mix by gentle rotation for 1 minute, taking care to avoid throwing soil up onto the sides of the flask. Let stand for 30 minutes.
5. Dilute to 200 ml. with deionized water.
6. Add 10 ml. phosphoric acid, 0.2g ammonium fluoride, and 10 drops diphenylamine indicator.
7. Titrate with 0.5N ferrous ammonium sulfate solution until the color changes from dull green to a turbid blue. Add the titrating solution drop by drop until the end point is reached when the color shifts to a brilliant green.
8. Prepare and titrate a blank in the same manner.
9. Prepare one duplicate sample and one quality control sample with each set of samples analyzed.
CALCULATION
1. % Organic Matter = 10[1(S÷B)] X 0.67
S = sample titration
B = blank titration
QUALITY CONTROL
1. Values on the duplicate samples must agree within 20% of the average of the two values.
2. Values on the quality control sample must lie within the limits established for this sample.
NOTE
1. This procedure requires the routine use of sulfuric acid. Sulfuric acid is a corrosive, strong oxidant and should be handled with caution. Refer to the MSDS sheet for proper handling.